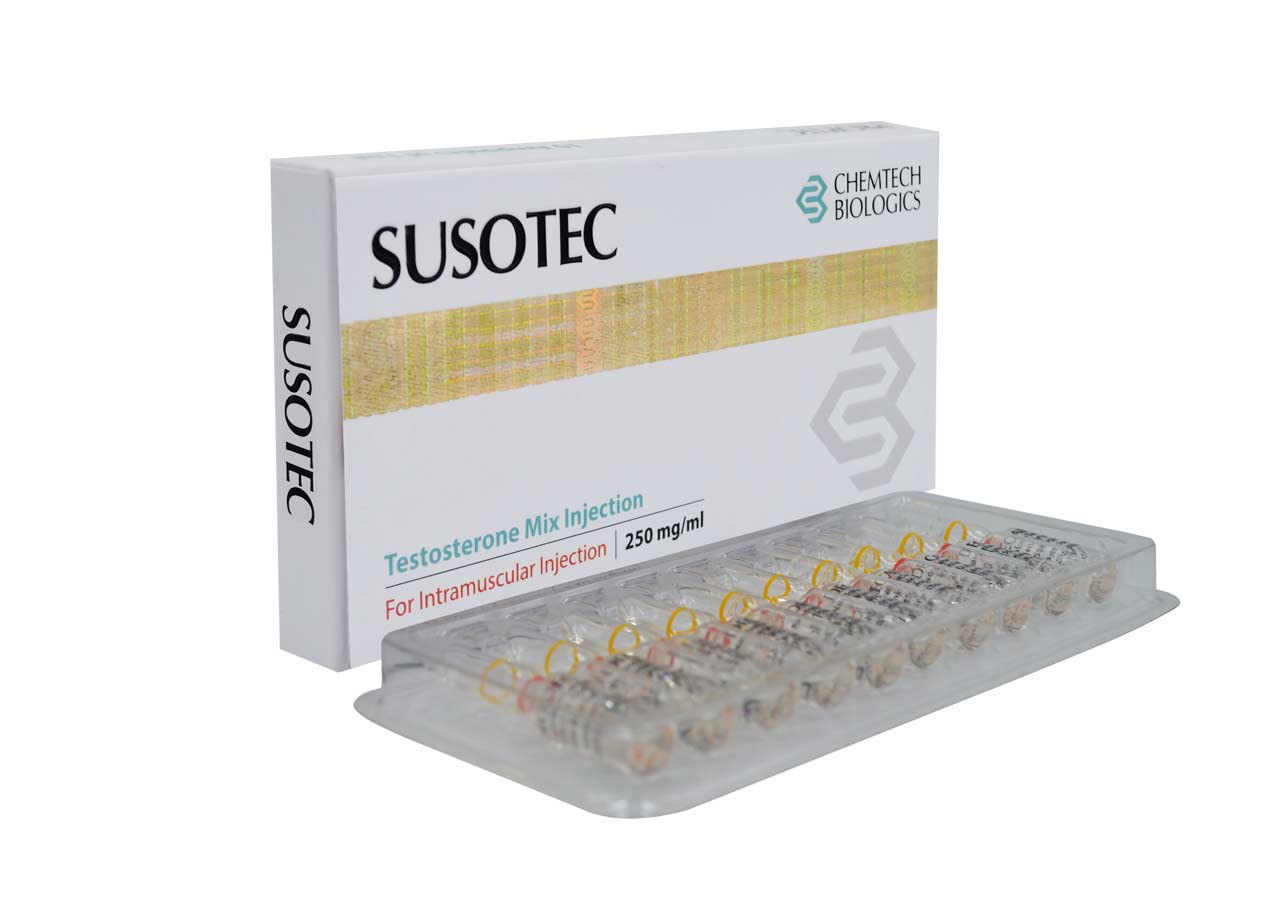
COMPOSITION:
Each ml contains:
Testosterone Decanoate BP 100 mg
Testosterone Isocaproate BP 60 mg
Testosterone Phenylpropionate BP 60 mg
Testosterone Propionate USP 30 mg Oil Base q.s.
INDICATIONS: Testosterone replacement therapy for male hypogonadism, when testosterone deficiency has been confirmed by clinical features and biochemical tests. Testosterone administration may also be used as supportive therapy for female-to-male transsexuals.
CONTRA-INDICATIONS: Pregnancy Known or suspected carcinoma of the prostate or breast Breastfeeding. Hypersensitivity to the active substance or to any of the excipients including arachis oil. Testosterone Mix Injection is therefore contraindicated in patients allergic to peanuts or soya.
DOSAGE AND DIRECTIONS FOR USE:
In general, the dose should be adjusted to the response of the individual patient. Adults (incl. elderly):
Usually, one injection of 1 ml per 3 weeks is adequate.
Paediatric population: Safety and efficacy have not been adequately determined in children and adolescents. Pre-pubertal children treated with Testosterone Mix Injection should be treated with caution. Female-to-male transsexuals: Different specialist centres have used doses varying from one injection of 1 ml every two weeks to one injection of 1 ml every four weeks. Method of administration Testosterone Mix Injection should be administered by deep intramuscular injection.
SIDE-EFFECTS AND SPECIAL PRECAUTIONS:
Due to the nature of Testosterone Mix Injection side effects cannot be quickly reversed by discontinuing the medication. Injectables in general may cause a local reaction at the injection site. synonyms and related terms.
Treatment in women: Treatment with Testosterone Mix Injection my induce signs of virilisation in women. Symptoms of virilisation may include hoarseness, acne, hirsutism, menstrual irregularity and alopecia.
Paediatric population: The following undesirable effects have been reported in prepubertal children using androgens: precocious sexual development, an increased frequency of erections, phallic enlargement and premature epiphyseal closure.
Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
Overdose: The acute toxicity of testosterone is low. If symptoms of chronic overdose occur (e.g. polycythaemia, priapism) treatment should be discontinued and after the disappearance of the symptoms, be resumed at a lower dosage.